Morphological Studies of Allotropes of Sulfur
Introduction
Sulfur, a non-metallic element with the symbol S and atomic number 16, exhibits a remarkable diversity in its allotropes. These allotropes, differing in molecular structure and physical properties, arise from the various ways sulfur atoms can bond and arrange themselves. Understanding the morphology of sulfur allotropes is crucial for applications in chemistry, materials science, and environmental studies. This essay delves into the morphological characteristics of sulfur’s allotropes, focusing on their structural forms, stability, and transitions.
1. Rhombic Sulfur (α-S₈)
Rhombic sulfur, also known as α-sulfur, is the most stable and commonly encountered allotrope at ambient conditions. It crystallizes in an orthorhombic system, forming yellow crystals that are insoluble in water but soluble in carbon disulfide. The molecular structure of α-sulfur consists of puckered S₈ rings, where each sulfur atom is bonded to two others, creating a crown-like shape. This allotrope is thermodynamically stable below 95.3°C and is widely used in various industrial applications due to its stability and ease of handling.
2. Monoclinic Sulfur (β-S₈)
Monoclinic sulfur, or β-sulfur, forms when rhombic sulfur is heated above 95.3°C. It crystallizes in a monoclinic system and is characterized by a higher density than α-sulfur. The molecular structure of β-sulfur also comprises S₈ rings, but the packing arrangement differs from that of α-sulfur, leading to distinct physical properties. β-sulfur is less stable than α-sulfur and gradually reverts to the rhombic form upon cooling. Its unique properties make it of interest in studies of phase transitions and crystallography.
3. Plastic Sulfur (Amorphous Sulfur)
Plastic sulfur, often referred to as amorphous sulfur, is obtained by rapidly cooling molten sulfur, preventing the formation of crystalline structures. This allotrope is characterized by long-chain polymeric sulfur molecules, which give it a rubber-like consistency. Plastic sulfur is unstable and undergoes a gradual transformation into rhombic sulfur over time. Its study provides insights into polymerization processes and the kinetics of phase transitions in sulfur.
4. Cyclo-Sulfur Allotropes
Cyclo-sulfur allotropes consist of sulfur molecules in ring structures with varying numbers of sulfur atoms. Notable examples include:
- Cyclo-S₆ (S₆): This allotrope features a six-membered ring of sulfur atoms and is one of the simplest cyclic sulfur molecules. It has been studied for its unique bonding and potential applications in chemical synthesis.
- Cyclo-S₈ (S₈): The most prevalent allotrope of sulfur, S₈ molecules form puckered rings and are the building blocks of rhombic and monoclinic sulfur. Their stability and abundance make them central to sulfur chemistry.
- Cyclo-S₁₀ and Higher Rings: Larger sulfur rings, such as S₁₀, S₁₂, and beyond, have been synthesized and characterized. These allotropes exhibit varying degrees of stability and have been explored for their potential in materials science and nanotechnology.
The study of cyclo-sulfur allotropes enhances our understanding of sulfur’s chemical versatility and its ability to form diverse molecular structures.
5. Polymeric Sulfur Allotropes
Polymeric sulfur allotropes are formed by the polymerization of sulfur molecules, resulting in long chains of sulfur atoms. These allotropes include:
- Catena-Sulfur: This form consists of linear chains of sulfur atoms and is typically observed in molten sulfur that has been rapidly cooled. The polymeric chains can vary in length and structure, influencing the material’s properties.
- Biradical Catena Chains: These chains feature sulfur atoms with unpaired electrons, leading to unique chemical reactivity and potential applications in chemical synthesis and materials science.
The morphology of polymeric sulfur allotropes is crucial for understanding sulfur’s role in various chemical processes and its potential in advanced materials.
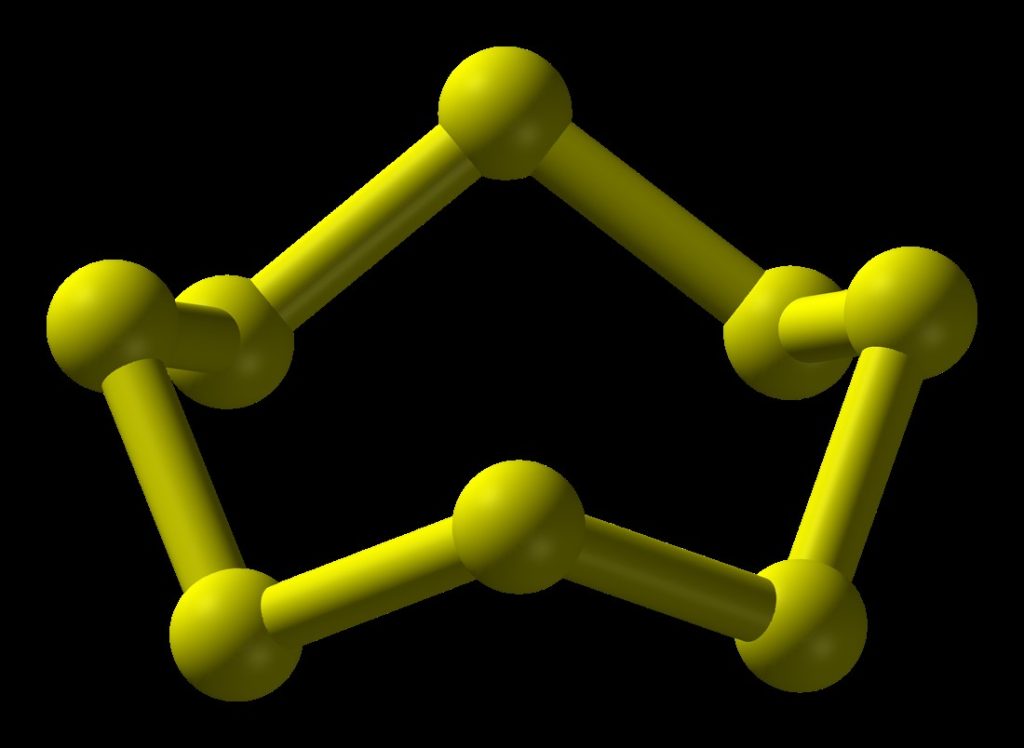
STRUCTUREOF CYCLOOCTASULPHUR MOLECULE ( S8)
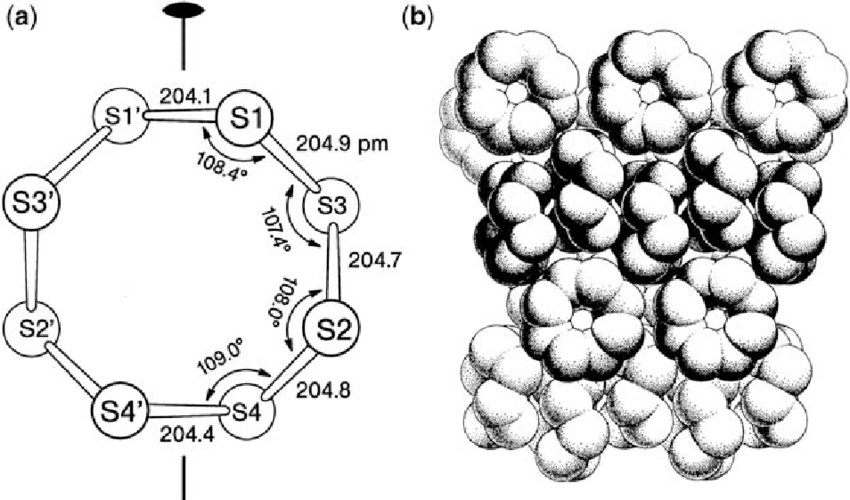
2 CRYSTAL AND ORTHORHOMBIC STRUCTURE OF SULPHUR(ALPHA S 8)
6. High-Pressure Sulfur Allotropes
Under high-pressure conditions, sulfur exhibits a variety of allotropes with distinct morphological characteristics:
- Metallic Sulfur: At pressures exceeding 10 GPa, sulfur undergoes a transition to a metallic state, characterized by a change in electronic structure and conductivity.
- Superconducting Sulfur: At extremely high pressures (above 200 GPa), sulfur has been observed to exhibit superconductivity, a phenomenon where it can conduct electricity without resistance.
These high-pressure allotropes are of significant interest in the study of condensed matter physics and materials science, as they challenge existing theories of elemental behavior under extreme conditions.
7. Sulfur Allotropes in Nature
In nature, sulfur exists in various allotropes, often in combination with other elements. These include:
- Sulfur in Biological Systems: Sulfur is a vital component of amino acids and vitamins, and its allotropes play roles in biological processes such as enzyme function and metabolism.
- Sulfur in Geological Formations: Sulfur deposits in the Earth’s crust exist in different allotropes, influencing the formation of minerals and the geochemical cycling of sulfur.
Understanding the natural occurrence and transformation of sulfur allotropes is essential for fields like biochemistry, geology, and environmental science.
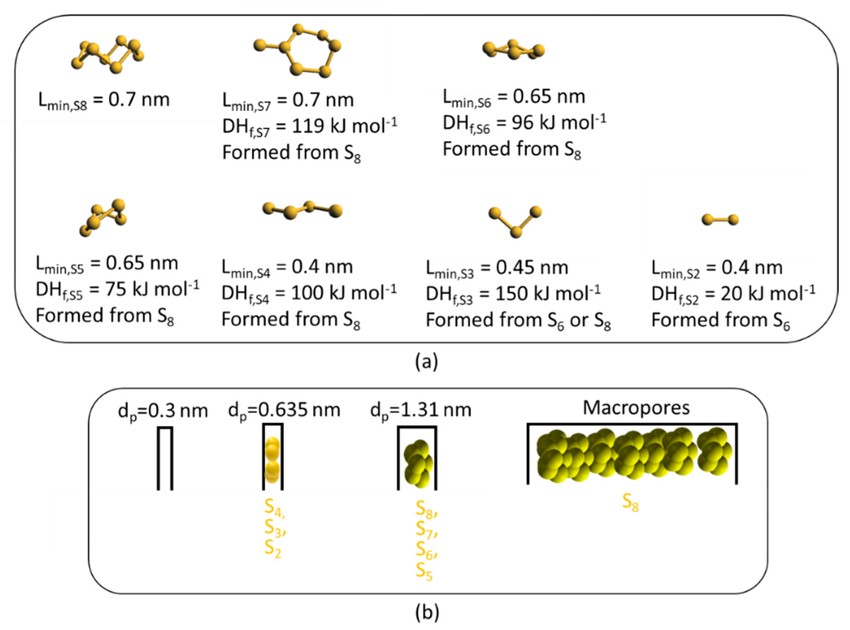
SUPLHUR ALLOTROPHS IN RELATION TO PORES OF A POROUS CATHODE HOST
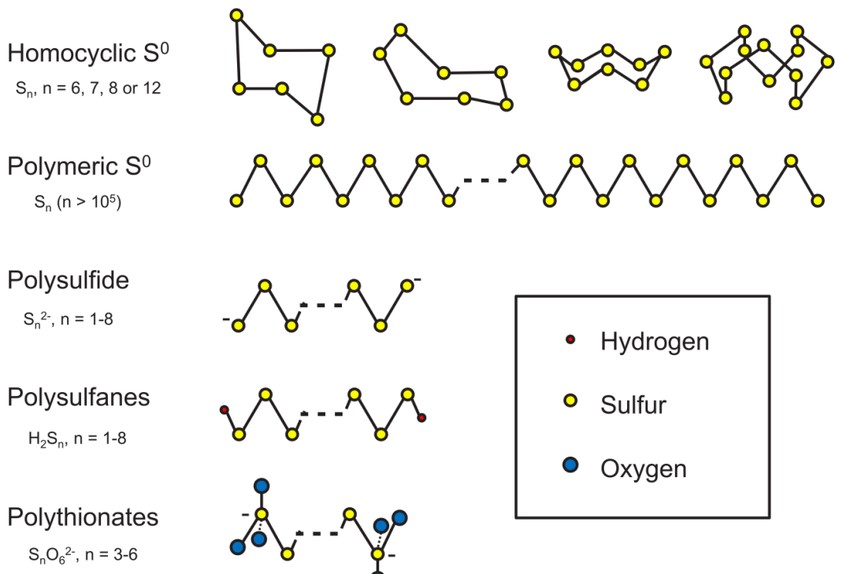
ALLOTROPES OF SULPHUR IN MICROBIAL RECYCLING OF THIS ELEMENT
8. Characterization Techniques
The study of sulfur allotropes requires advanced characterization techniques:
- X-ray Crystallography: This method provides detailed information about the atomic arrangement in crystalline sulfur allotropes, aiding in the determination of their molecular structures.
- Spectroscopic Methods: Techniques such as infrared spectroscopy and Raman spectroscopy are employed to investigate the vibrational modes and bonding characteristics of sulfur molecules.
- Electron Microscopy: Scanning and transmission electron microscopy allow for the observation of sulfur’s morphology at the nanoscale, revealing details about crystal shapes and sizes.
These techniques are indispensable for elucidating the properties and behaviors of sulfur allotropes.
9. Applications of Sulfur Allotropes
The diverse allotropes of sulfur have a wide range of applications:
- Industrial Applications: Sulfur is used in the production of sulfuric acid, vulcanization of rubber, and as a fungicide in agriculture.
- Energy Storage: Certain sulfur allotropes are being explored for their potential use in lithium-sulfur batteries, offering high energy densities.
- Pharmaceuticals: Sulfur compounds are utilized in the synthesis of various drugs and in the treatment of skin conditions.
- Environmental Science: Understanding sulfur allotropes aids in the study of sulfur cycles in ecosystems and the development of methods to mitigate sulfur-related pollution.

10. Conclusion
Sulfur’s ability to form a multitude of allotropes with distinct morphological characteristics underscores its chemical versatility and importance. The study of these allotropes provides valuable insights into sulfur’s behavior under various conditions and its applications across different fields. Continued research into the morphology of sulfur allotropes will enhance our understanding and open new avenues for technological advancements.
References
- Steudel, R. (2003). Sulfur: A New Perspective. Wiley-VCH.
- Meyer, B. (1964). Solid Allotropes of Sulfur. Chemical Reviews, 64(1), 1-24.
- Kitagawa, H., et al. (2016). Isolation and evolution of labile sulfur allotropes via kinetic trapping. Nature Communications, 7, 11856.
- Ferrari, P., et al. (2024). Laboratory infrared spectra and fragmentation chemistry of sulfur allotropes. Nature Communications, 15(1), 1234.
- Tarasova, N. P., et al. (2021). Elemental sulphur in the synthesis of sulphur-containing compounds. Nature Communications, 12(1), 8695231.
- “Allotropes of sulfur.” (2025). Wikipedia. Retrieved from https://en.wikipedia.org/wiki/Allotropes_of_sulfur
- “Sulfur Properties.” (2025). Georgia Gulf Sulfur. Retrieved from https://georgiagulfsulfur.com/ggs/old_site/properties.htm
- “Sulfur’s Amazing Structure.” (2025). MEL Chemistry. Retrieved from https://melscience.com/US-en/articles/sulfurs-amazing-structure/
- “Sulfur Allotropes in Relation to Pores in Porous Cathode Host.” (2021). Journal of Composites Science. Retrieved from https://www.researchgate.net/figure/Sulfur-allotropes-in-relation-to-pores-in-porous-cathode-host-a-molecular-models-of_fig3_349631879
- “2 Crystal and Molecular Structure of Orthorhombic α-S₈.” (2020). ResearchGate. Retrieved from https://www.researchgate.net/figure/Crystal-and-molecular-structure-of-orthorhombic-a-S-8-the-stable-allotrope-of-sulfur-at_fig2_344809835
- “Allotropes of Sulfur – Wikipedia.” (2025). Wikipedia. Retrieved from https://en.wikipedia.org/wiki/Allotropes_of_sulfur
- “Allotropy of Sulfur – Solid State Chemistry @Aalto.” (2025). Aalto University. Retrieved from https://wiki.aalto.fi/display/SSC/Allotropy%2Bof%2Bsulfur
- “A Primer on Sulfur for the Planetary Geologist.” (2025). NASA Astrobiology Institute. Retrieved from https://rgcps.asu.edu/nasa-pdfs/PrimerOnSulfurForThePlanetaryGeologist.pdf
- “Sulfur Allotrope Chemistry.” (2001). eScholarship@McGill. Retrieved from https://escholarship.mcgill.ca/downloads/g732db63z.pdf
- “Sulfur Properties.” (2025). Georgia Gulf Sulfur. Retrieved from https://georgiagulfsulfur.com/ggs/old_site/properties.htm
- “Sulfur’s Amazing Structure.” (2025). MEL Chemistry. Retrieved from https://melscience.com/US-en/articles/sulfurs-amazing-structure/
- “Sulfur Allotropes in Relation to Pores in Porous Cathode Host.” (2021). Journal of Composites Science. Retrieved from https://www.researchgate.net/figure/Sulfur-allotropes-in-relation-to-pores-in-porous-cathode-host-a-molecular-models-of_fig3_349631879
