The Morphology of Carbon: A Comprehensive Exploration of Its Allotropes
Introduction
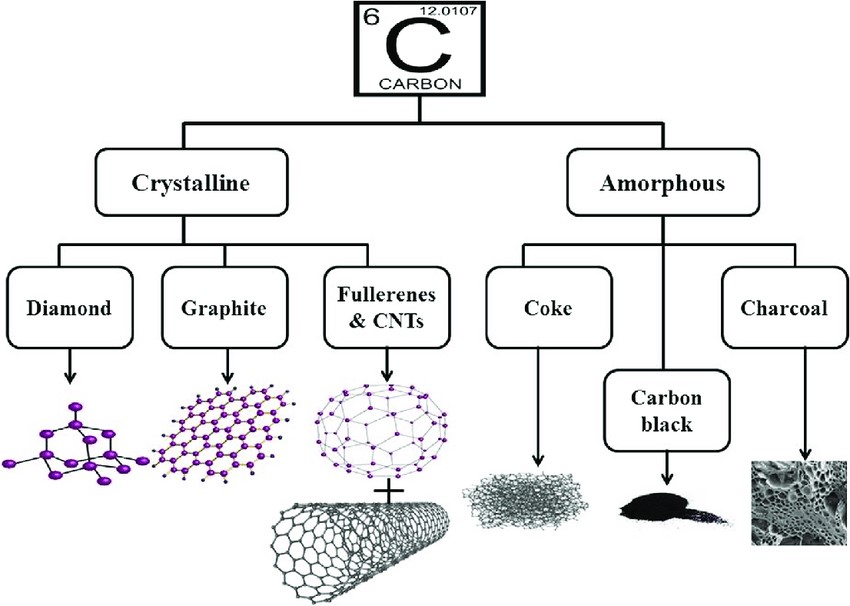
Carbon, the fourth most abundant element in the universe by mass, is renowned for its unparalleled versatility in forming a myriad of allotropes. These allotropes—distinct structural forms of the same element—arise from the unique ways carbon atoms bond and arrange themselves. The study of carbon’s morphology, which refers to the form and structure of these allotropes, is crucial for understanding their properties and potential applications. This essay delves into the morphology of carbon, examining its most prominent allotropes: diamond, graphite, graphene, fullerenes, carbon nanotubes, and amorphous carbon.
Diamond: The Tetrahedral Marvel
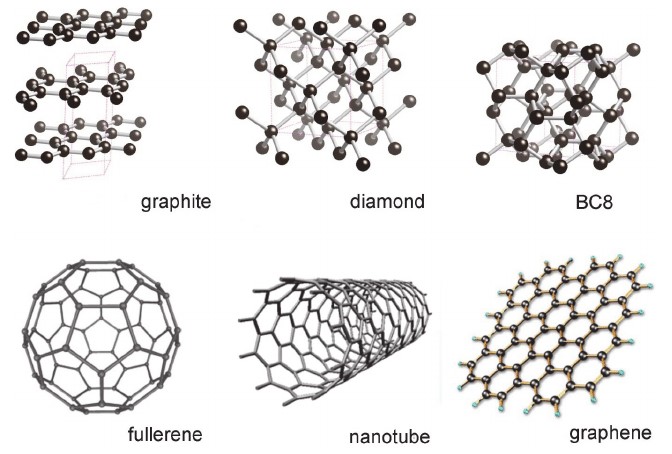
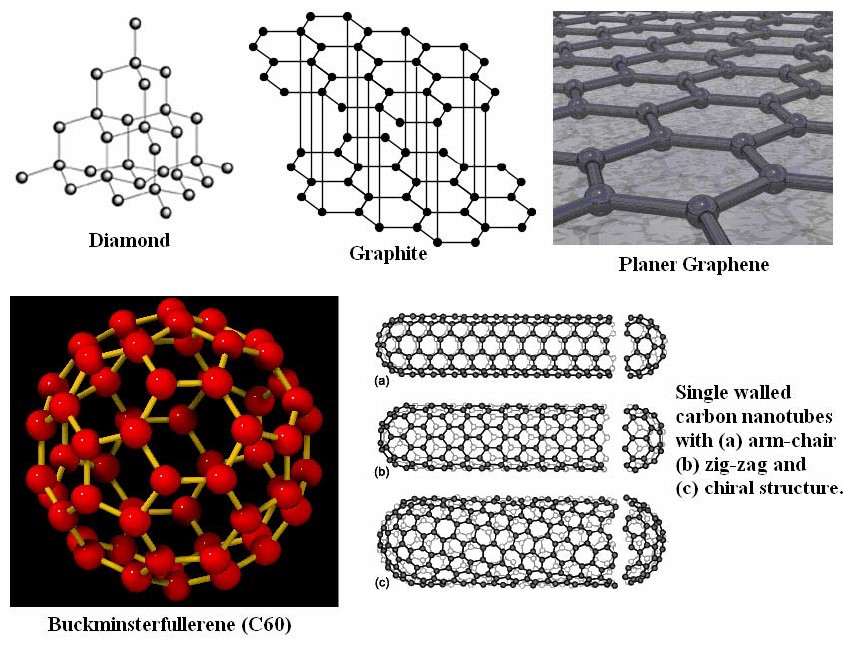
Diamond is perhaps the most iconic allotrope of carbon, celebrated for its exceptional hardness and brilliance. Its morphology is characterized by a three-dimensional tetrahedral lattice structure, where each carbon atom is covalently bonded to four other carbon atoms in a rigid, repeating pattern. This sp³ hybridization results in a crystal lattice that is both strong and transparent, contributing to diamond’s renowned optical properties. The hardness of diamond makes it invaluable in cutting tools and abrasives, while its transparency and brilliance have made it a symbol of luxury in jewelry.
Graphite: The Layered Conductor
Graphite presents a stark contrast to diamond in its morphology. Composed of planar sheets of carbon atoms arranged in a hexagonal lattice, graphite exhibits sp² hybridization. These sheets are stacked loosely, held together by weak van der Waals forces, allowing them to slide over one another easily. This unique structure imparts to graphite its characteristic lubricity and electrical conductivity, as the delocalized π-electrons within the layers facilitate electron flow. Graphite’s morphology makes it ideal for applications such as lubricants, batteries, and electrodes.
Graphene: The Single Layered Wonder
Graphene is a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice. It can be considered the fundamental building block of other carbon allotropes like graphite, carbon nanotubes, and fullerenes. Graphene’s morphology endows it with remarkable properties, including exceptional electrical conductivity, mechanical strength, and thermal stability. These attributes make graphene a promising material for a wide range of applications, from flexible electronics to high-capacity batteries.
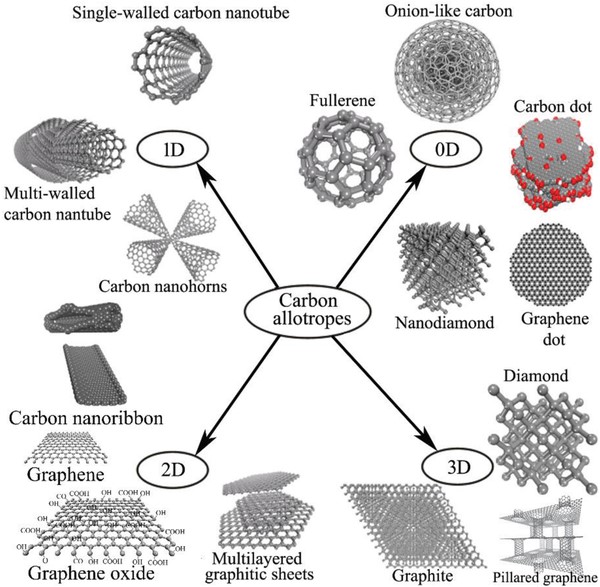
Fullerenes: The Molecular Spheres
Fullerenes are a class of carbon allotropes where carbon atoms are arranged in spherical, ellipsoidal, or tubular forms. The most well-known fullerene is C₆₀, also known as buckminsterfullerene or “buckyball,” which resembles a soccer ball in shape. Fullerenes exhibit unique morphological features, such as curvature and closed-cage structures, which impart distinct chemical and physical properties compared to other carbon allotropes. These properties make fullerenes suitable for applications in drug delivery systems, photovoltaics, and as catalysts in chemical reactions.
Carbon Nanotubes: The Cylindrical Nanostructures
Carbon nanotubes (CNTs) are cylindrical structures composed of rolled-up sheets of graphene. They can be classified into single-walled (SWCNTs) and multi-walled carbon nanotubes (MWCNTs), depending on the number of graphene layers. The morphology of CNTs, characterized by their high aspect ratio and unique electronic properties, makes them ideal candidates for applications in nanocomposites, sensors, and nanoelectronics. The alignment and chirality of the graphene sheets in CNTs significantly influence their electrical properties, allowing for the tailoring of materials for specific applications.
Amorphous Carbon: The Disordered Network
Amorphous carbon lacks a long-range ordered structure, distinguishing it from crystalline allotropes like diamond and graphite. Its morphology is characterized by a disordered network of carbon atoms, which can exhibit varying degrees of sp² and sp³ hybridization. This lack of order imparts to amorphous carbon unique properties, such as high surface area and tunable electronic characteristics. Amorphous carbon is utilized in various applications, including thin-film coatings, solar cells, and as a precursor for the synthesis of other carbon nanomaterials.
Comparative Analysis of Carbon Allotropes
Allotrope | Hybridization | Morphological Feature | Notable Property | Common Applications |
Diamond | sp³ | Tetrahedral lattice | Hardness, transparency | Cutting tools, jewelry |
Graphite | sp² | Layered hexagonal sheets | Lubricity, electrical conductivity | Batteries, lubricants, electrodes |
Graphene | sp² | Single-layered honeycomb lattice | High strength, conductivity | Flexible electronics, sensors |
Fullerenes | sp² | Spherical/ellipsoidal cages | Unique chemical reactivity | Drug delivery, photovoltaics |
Carbon Nanotubes | sp² | Cylindrical tubes | High aspect ratio, conductivity | Nanocomposites, sensors |
Amorphous Carbon | sp²/sp³ | Disordered network | High surface area, tunable properties | Thin-film coatings, solar cells |
Characterization Techniques
Understanding the morphology of carbon allotropes requires advanced characterization techniques:
- Scanning Electron Microscopy (SEM): Provides high-resolution images of surface morphology, aiding in the examination of nanostructures.
- Transmission Electron Microscopy (TEM): Allows for the observation of internal structures at the atomic level, essential for studying the arrangement of carbon atoms in nanomaterials.
- Raman Spectroscopy: Detects vibrational modes in carbon materials, providing insights into the degree of crystallinity and the presence of defects.
- X-ray Diffraction (XRD): Analyzes the crystalline structure of carbon allotropes, helping to identify phases and orientations.
- Atomic Force Microscopy (AFM): Measures surface topography at the nanoscale, useful for assessing roughness and elasticity.
Applications and Future Directions
The diverse morphologies of carbon allotropes enable a wide range of applications:
- Electronics: Graphene and CNTs are explored for next-generation transistors and flexible electronic devices due to their exceptional conductivity and mechanical properties.
- Energy Storage: Graphene and CNTs are utilized in supercapacitors and batteries for their high surface area and conductivity, enhancing energy storage capabilities.
- Nanocomposites: Carbon nanomaterials are incorporated into polymers to improve strength, conductivity, and thermal stability.
- Biomedical Applications: Fullerenes and CNTs are investigated for drug delivery systems, owing to their ability to encapsulate molecules and target specific cells.
Future research may focus on the synthesis of novel carbon allotropes, such as penta-graphene and carbon nanothreads, which promise unique properties and potential applications in various fields.
Conclusion
Carbon’s ability to form a vast array of allotropes with distinct morphologies underscores its central role in materials science and technology. From the hardness of diamond to the flexibility of graphene, the diverse structures of carbon materials offer a plethora of possibilities for innovation. Continued exploration and understanding of carbon’s morphology will undoubtedly lead to advancements in numerous technological domains.
References
- Wikipedia contributors. (2025). Allotropes of carbon. In Wikipedia, The Free Encyclopedia. Retrieved from https://en.wikipedia.org/wiki/Allotropes_of_carbon
- Open Access Journals. (2025). The Morphology of Carbon: An Exploration of Forms and Structures. Open Access Journals. Retrieved from https://www.openaccessjournals.com/articles/the-morphology-of-carbon-an-exploration-of-forms-and-structures-18074.html
- ScienceDirect. (2025). Describing carbons. ScienceDirect. Retrieved from https://www.sciencedirect.com/science/article/pii/S2667056924000063
- PubMed Central. (2025). Carbon-Based Nanomaterials/Allotropes: A Glimpse of the Future. PubMed Central. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC5848992/
- LibreTexts. (2025). The Group 14 Elements and the Many Allotropes of Carbon. LibreTexts Chemistry. Retrieved from https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Inorganic_Chemistry_(LibreTexts)/8%3A_Chemistry_of_the_Main_Group_Elements/8.07%3A_Group_14/8.7.01%3A_The_Group_14_Elements_and_the_many_Allotropes_of_Carbon
- American Chemical Society. (2015). Broad Family of Carbon Nanoallotropes: Classification and Properties. ACS Publications. Retrieved from https://pubs.acs.org/doi/10.1021/cr500304f
Byju’s. (2025). Allotropes of Carbon. Byju’s. Retrieved from https://byjus.com
